Usp General Chapter 1116
Products not required to be sterile. Acceptance Criteria for Microbiological Quality of Non-Low water activity has traditionally been used to.

Lb3o5tewmnyggm
The purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug substances dosage forms and in certain cases medical devices.

. In general organ-isms with relatedness less than or equal to 97 are consid-ered different genera and those with relatedness less than or equal to 99 are considered different. The United States Pharmacopeia USP Micro biological Control and Monitoring of Aseptic Process ing Environments 1 marks a significant shift in ABOUT THE AUTHOR. 1 Pharmaceutical sterile products 2.
USP-NF standards and specifications for drugs are also recognized and adopted internationally. May not be used as substitutes for official titles. Page 1 of 22.
The scope of USP has been narrowed to apply to the following products manufactured in an aseptic processing environment. This general information chapter provides information and recommendations for. The recently revised United States Pharmacopoeia USP chapter Microbial Control and Monitoring of Aseptic Processing Environments includes a thorough description definition and.
The purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug substances dosage forms and in certain cases medical devices. Accessed from 67851037 by clinical6 on Sun Aug 25 160327 EDT 2013 USP 36 General Information 1116 Aseptic Processing Environments 787 meaningful process. Up to 3 cash back 2017 USPC Official 12116 - 043017 General Chapters.
The recently revised United States Pharmacopoeia USP chapter Microbiological Control and Monitoring of Aseptic Processing Environments includes a. The purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug substances dosage forms and in certain cases medical devices. 1116 MICROBIOLOGICAL CONTROL AND MONITORING.
Following are the details of the revision. In the Chapter. Microbiologically controlled environments are used for a variety of purposes within the healthcare industry.
Compounded medications made without. USP Official Reference Standards USP offers over 7000 USP Reference Standards highly characterized physical specimens of drug substances excipients food ingredients impurities. 21 CFR 211113 arequires written procedures directed to control of objectionable microorganisms in drug.
692 1111 Microbiological Examination General Information USP 35 Table 2. Chapter is arguably one of the most comprehensive informational chapters from the USP and it is particularly challenging due to its proposal regarding. Pharmacopeia USP and the National Formulary NF.
Up to 3 cash back intended use. Requirements stated in these General Notices apply to all articles Official articles include. Up to 3 cash back Usp 43 General Information 1116 7833 OVERALL MANAGEMENT OF A MICROBIOLOGICAL CONTROL PROGRAM The management ofa succesful microbiological.
The General ChaptersMicrobiology Expert Committee is responsible for developing new and revising existing general chapters related to microbiology and sterility assurance. Understanding the risks inherent in sterile compounding and incorporating established standards are essential for patient safety.

Biotrends Tm

Lb3o5tewmnyggm
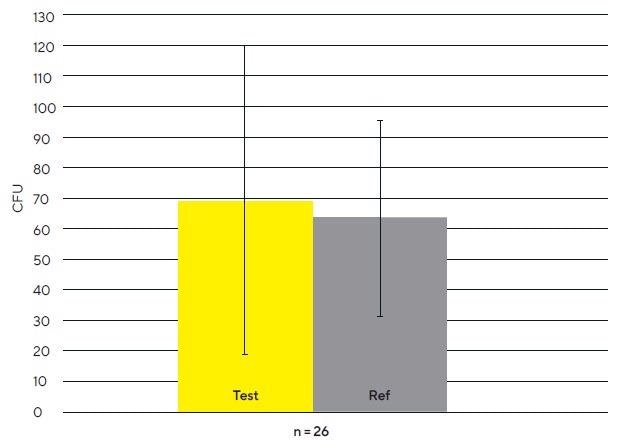
Microbial Air Monitoring In Clean Room Environments

Usp 1116 Pdf Environmental Monitoring Sterilization Microbiology

Ejpps Article

2020 Partner S Instructions For Schedule K 1 Form 1065 1116 Pdf4pro

Designing An Environmental Monitoring Program For Non Sterile Manufacturing A Risk Based Approach

Environmental Monitoring Considerations Ppt Download
Current Activities Of The Usp Microbiology Expert Committee European Pharmaceutical Review

Biotrends Tm

Mikrobiologisches Monitoring Von Aseptischen Prozessen Pdf Free Download

Download Usp General Chapter Us Pharmacopeial

Microbiological Monitoring During Aseptic Handling Methods Limits And Interpretation Of Results Sciencedirect

Pdf New Guidance For Environmental Monitoring In Cleanrooms

Usp 36 Chapter 1116 Environment Monitoring
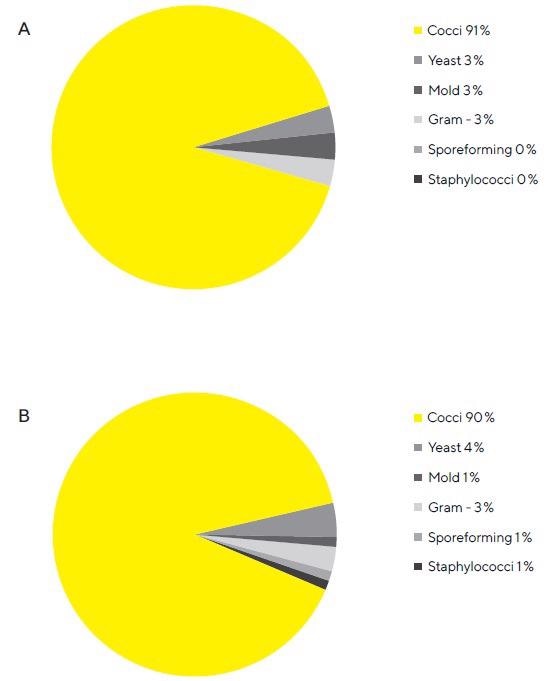
Microbial Air Monitoring In Clean Room Environments

Lifecycle Management For Near Sterile Facility Contamination Control Programs American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology